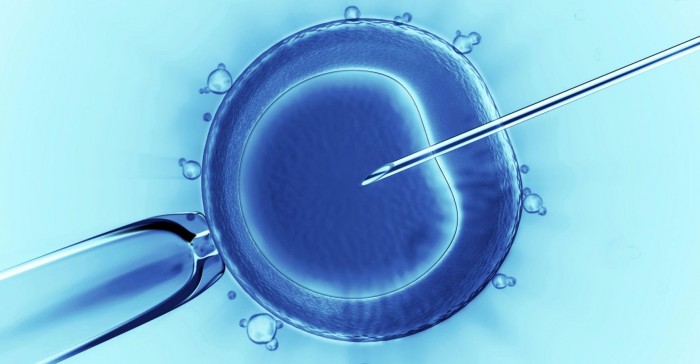
In April of 2015, researchers in China from Sun Yat-sen University published the results of the world’s first experiment on human embryo editing. The goal of the experiment was to edit a gene containing mutations for a blood disorder called β-thalassemia. The gene is responsible for coding a subunit of the hemoglobin molecule, which carries oxygen through the bloodstream.
The announcement resurfaced past questions regarding the ethics of manipulating human reproduction at the embryonic level.
“[That experiment was] the first step in a well mapped-out process leading to GM [Genetically Modified]-babies, and a future of consumer eugenics,” David King, director of Human Genetics Alert, a UK-based NGO and human genetics watchdog group, stated in an interview with the BBC.
In an attempt to pacify the ethical issues surrounding the use of human embryos, the ones used by the Chinese researchers contained three sets of chromosomes, as opposed to the usual two sets. This was the result of induced errors during the in-vitro fertilization (IVF) process. These embryos can divide into a blastula, or a bundle of 200 to 300 cells, but cannot develop fully into a fetus.
Out of the 86 embryos used, only 28 were successfully spliced, or genetically modified. After a closer analysis of the genetic makeup of the spliced embryos, the researchers found an alarming number of side mutations on other unintended targets. This is one of the main ethical concerns associated with genetic editing, since the mutations accrued in an embryo would be present in all the cells of the body as it divides.
“I believe this is the first [experiment] applied to human pre-implantation embryos and as such the study is a landmark, as well as a cautionary tale,” George Daley a stem cell biologist from Harvard Medical School said in an interview with Nature. “Their study should be a stern warning to any practitioner who thinks the technology is ready for testing to eradicate disease genes.”
In September 2015, only months after this first experiment in April, the UK’s governmental authority on human fertilization and embryology received a proposal from the Francis Crick Institute in London for another experiment involving human embryonic gene editing. On Feb. 4, the experiment was approved.
Dr. Kathy Niakan, the stem cell biologist leading the experiment, and her team plan to begin the study as soon as possible. Niakan hopes to determine which genes are involved in cell type and tissue differentiation in the first few days of human development using a gene editing technique called CRISPR/Cas9, the same technique the Chinese researchers used in their experiment.
CRISPR/Cas9 is a genetic editing technique used in molecular biology to study the functions of proteins, as well as how they interact with one another. This can be used for both gene editing, as was the case in the Sun Yat-sen University experiment, as well as gene deletion, known as gene knockdown, which is the technique proposed by Niakan.
The mechanisms of the initial stages of human development are not well understood, and the human embryo is notoriously inefficient, with 31 per cent of all pregnancies ending in miscarriage. Genetic knockdown studies are used to determine the effects of a gene by essentially removing it from the body and then comparing the knock-down to the control, where no genes were removed. Genetic knockdowns are easier to perform than genetic editing because it requires less precision, with the only goal being to ‘break’ the gene in question.
Generally, knockdown screens are used to target a specific problem like what is causing individuals to suffer from β-thalassemia. Genetic editing, however, can be used to evaluate a broad range of situations, such as embryonic development. Niakan and her team aren’t trying to answer a single question, they’re just trying to understand human development by looking at it in a variety of ways using CRISPR/Cas9.
“The research could lead to improvements in fertility treatment and a better understanding of the first stages of life,” Niakan said at a press briefing in London.
The embryos will come from fertility clinic patients across the UK. Following a course of in-vitro fertilization (IVF) treatment, extra embryos are usually generated. With the informed consent of the patient, Niakan hopes to uses some of these embryos in her research, with the understanding that they will be destroyed after only seven days.
Niakan hopes that her research will pave the way for more successful treatment possibilities for infertility in the future.