Pulmonary infections—such as COVID-19 and the flu—in which bacteria or viruses enter and damage the lungs, are among the leading causes of death in older adults. Elderly people’s increased susceptibility to pulmonary infections is attributed mostly to immune systems that weaken over time.
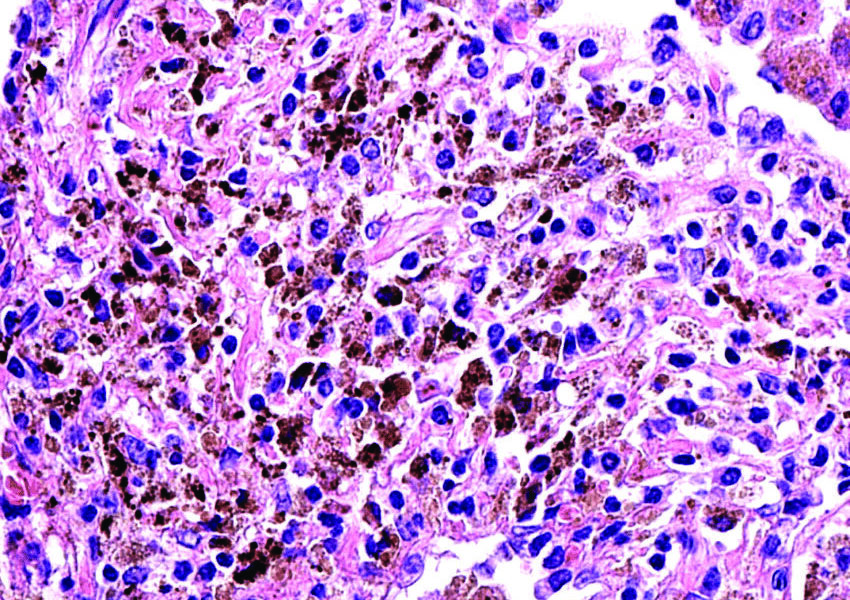
The initiation of immune responses to protect against lung damage involves a key group of players known as alveolar macrophages (AMs). Recently, McGill researchers discerned previously unknown mechanisms behind AM growth and longevity. Their recently published study provides important insights into the processes that affect the self-proliferation of AMs and the intricate connections between neonatal neutrophils—white blood cells found in newborns—and AMs.
AMs are a type of tissue-resident macrophage, an immune cell that takes on diverse roles, including blood vessel formation, bone degradation, and activation of immune cells. Tissue-resident macrophages are found in the tissues of various organs, such as the brain and intestines, and perform tissue-specific functions. Alveoli, the tiny air sacs in the lungs where gas exchange of oxygen and carbon dioxide occurs, contain AMs.
“AMs are critical for pulmonary homeostasis and immunosurveillance, and they can both initiate the immune response and the resolution of inflammation,” Maziar Divangahi, a professor of medicine at McGill whose lab led the study, wrote in an email to //The McGill Tribune//. “For example, AMs are the first immune cells encountered in the airways by respiratory viruses, such as SARS-CoV-2, and contribute to viral clearance, limiting lung damage and promoting tissue healing.”
AMs also help remove excess surfactant, thereby maintaining lung homeostasis—the proper functioning of the lungs. Lung surfactants are materials produced by alveolar cells that prevent the collapse of alveolar walls when exhaling; they are essential to normal lung function. But, when an excessive amount of surfactant is produced by alveolar cells or when AMs fail to remove the surfactant, the gas exchange that takes place in alveolar walls can be significantly hindered, making breathing more difficult.
“AMs are crucial for many aspects of lung immunity, and they represent an excellent target for promoting healthy lungs,” Divangahi wrote. During embryonic development, AMs develop from fetal monocytes, which are white blood cells derived from the bone marrow and precursors to macrophages. Upon maturation, AMs self-renew throughout their lifespan, contributing to their remarkable longevity in the lungs.
In the new paper, Divangahi and his team found that the absence of 12-HETE, a derivative of fat molecules, led to a significant reduction in AMs in the lungs. This reduction was attributed to elevated levels of prostaglandin E2—a ubiquitous lipid mediator.
The paper also explored variations in immune cell functions in different stages of life.
“While adult neutrophils, [a type of white blood cell that fights infection], don’t express 12-HETE, our study shows that the neonatal neutrophils do,” Divangahi wrote. “This highlights the fact that the function of immune cells in neonates versus in adults is diverse, which is incompletely understood.”
In other words, excess prostaglandin E2 production acts as a brake on the proliferative self-renewal of AMs, impairing immune function. The impaired immune function characterized by reduced numbers of AMs could increase the risk of acute lung injuries and lung infections, such as influenza A virus and SARS-CoV-2.
As researchers delve deeper into understanding AM mechanisms, Divangahi envisions these cells to be a revolutionary panacea in lung health and medical intervention.