Parkinson’s disease affects the dopamine neurons in as many as ten million people worldwide yet, to this day, nobody has identified a concrete cause. However, science may be a step closer, as researchers have recently shown that the protein alpha synuclein detrimentally affects the brains of Parkinson’s patients. Scientists have long suspected that alpha synuclein plays a role in the disease and this discovery opens up new research avenues to better understand it.
During her presentation at the Science Undergraduate Society’s Academia Week, Nguyen-Vi Mohamed, a postdoctoral fellow at the Montreal Neurological Institute, presented her lab’s work on the future of disease modelling: Growing realistic 3D organs.
Mohamed’s lab seeks to better understand the propagation of alpha-synuclein to investigate the pathological mechanisms that drive Parkinson’s disease. To do so, they follow a simple recipe to reprogram adult cells, credited to 2012 Nobel Prize for Medicine winner Shinya Yamanaka. The novel method takes blood samples from individuals with a genetic predisposition to Parkinson’s and uses them to generate stem cells, which can potentially become any cell of the human body.
“The development of [3D organs is] a major technological advance in the stem cell field,” Mohamed said. “This model [represents] a bridge between traditional 2D culture and the […] mouse model.”
Scientific researchers have been using mice for more than one hundred years because they are genetically similar to humans, as well as an array of other organisms. However, human biology is essential to fully understanding and treating medical conditions. Another popular option for research are cells grown in 2D cell cultures. Although they are human cells, these 2D cultures are still different from natural biological systems because they grow in a flat layer on a plastic surface.
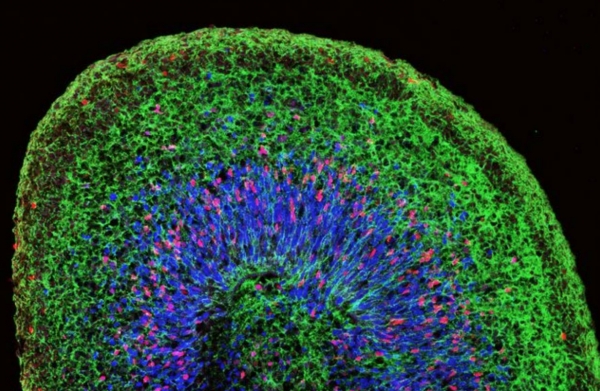
Researchers like Mohamed are increasingly looking into 3D cultures, which produce organs that can attach organically to one another. These 3D culture organs, or organoids, can grow in as little as 50 days to a diameter of four millimetres. Their increased complexity allows for a wide range of cell types that researchers can observe to determine their involvement in pathologies like Parkinson’s.
The organ most relevant to grow for the study of Parkinson’s is the brain. Mohamed’s work on creating small, human brain-like organs joins other recent research, including a 2013 study outlining the first cerebral organoid model. Researchers soon followed this development in 2016 with the formation of an organoid model specific to the midbrain, a region where dopaminergic neurons, which are lost in patients with Parkinson’s, are found in abundance.
Future work will focus on the fusion between a mini-brain derived from a patient with Parkinson’s and that of a healthy individual to study the effects of alpha synuclein in a healthy system. More broadly, the formation of a fully-functional brain in absence of a body opens doors to the study of other neurological conditions, such as Alzheimer’s and autism.
Mohamed’s ultimate goal is to integrate her model into drug-screening platforms. The future of pharmacological research exists at the intersection between traditional methods and these newer 3D models.
In addition to gender, ethnicity, and genetics, one of the risk factors for Parkinson’s disease is age. Since mini-brains have the capacity to age naturally, Mohamed hopes to make scientific breakthroughs in organoid ageing.
“It’s [an] exceptional kind of work,” Karina Paliotti (U3 Science) said. “I think it’s really cool [and] I think there’s a lot of hope [that] a lot of [good could] come out of it in the future.”