Besides acting as the backbone of genetic material, DNA is getting significant attention for being a versatile building block of nanomaterials—particles one-thousandth of the diameter of a hair strand—including a type of nanomaterial called DNA hydrogels.
As a rising star in the field of nanoengineering, which is the study of extremely small-scale particles, these hydrogels have great potential for biomedical uses. In a recent paper published in Advanced Science, McGill researchers proposed the use of a novel structure that would allow the formation of stronger DNA hydrogels for wider biomedical applications, such as enhancing drug efficacy.
Hydrogels are structures that can hold large amounts of water. For example, some hydrogels can absorb up to 600 times their original volume of water. They can be prepared from protein molecules, such as collagen and gelatin, and carbohydrate molecules, such as starch and agarose. DNA’s ability to absorb water enables it to possess the properties of a hydrogel.
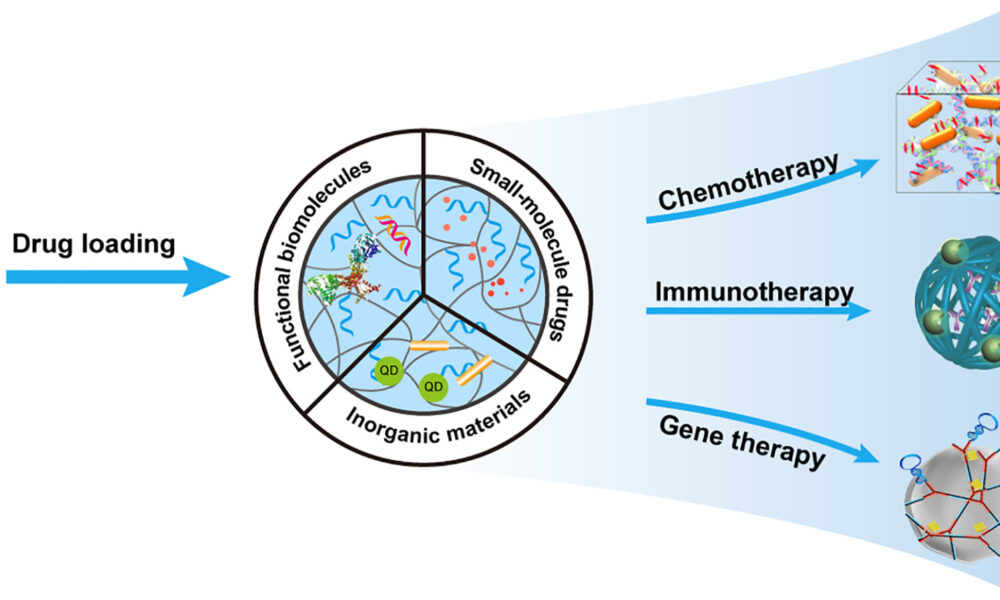
DNA-based hydrogels, which are composed entirely of DNA, can be produced through chemical or physical linkages between DNA strands. They exhibit various sought-after qualities, such as biodegradability, the ability to self-heal, and non-toxicity, making them an ideal choice for in-body tasks such as drug delivery, targeted gene therapy, and cancer treatment.
However, DNA hydrogels are not without their drawbacks. For instance, hydrogels made of unmodified DNA are extremely soft, making them incompatible with certain environments, such as the spleen, which has stiffer cells.
Additionally, unmodified DNA hydrogels lack a convenient chemical handle, so they cannot be used for specific biomedical applications, such as controlled drug delivery, tissue engineering, and cell transplant therapy. Chemical strategies for the construction of hydrogels, notably ligase-mediated reactions, allow the hydrogels to be fine-tuned and tailored for these kinds of applications.
“Scientists who wish to combine the attributes of DNA with say, catalysis, drug delivery, photochemistry, cell growth, or any other purpose for which chemistry has many solutions, rely on synthetically modified DNA,” wrote Christophe Lachance-Brais, a PhD student in McGill’s Department of Chemistry who led the study, in an email to The McGill Tribune. “Without a convenient chemical handle, DNA hydrogels are limited to the chemistry of DNA, and that may not be enough.”
In other words, a convenient chemical handle allows biomaterials such as DNA to be re-constructed for biomedical applications.
Lachance-Brais and his team proposed the use of a novel nucleic acid structure resulting from a genetic assembly called a dA/CA motif, which is made up of poly-deoxyadenosine (dA)—a derivative of DNA components—and cyanuric acid (CA)—a small non-toxic molecule.
“Our hydrogels could theoretically load up one molecule per adenine base, while the ones incorporating the molecule as low-density synthetic modifications could only release one per strand,” Lachance-Brais wrote. In other words, the new hydrogels are high-density, allowing them to have a high drug-loading capacity. This is advantageous because it means fewer hydrogels could be used to deliver the same amount of medicine.
This novel DNA hydrogel can also respond to complex stimuli, including specific DNA sequences and small molecules. When the hydrogels come into contact with a stimulus, such as a drug solution, they swell up to absorb the drug.
The new hydrogel can even influence gene expression by delivering a high concentration of gene-related therapeutics. This is done by enhancing the gene-silencing efficacy of antisense oligonucleotides—a versatile group of therapeutics with gene-silencing effects used to treat diseases such as Duchenne muscular dystrophy and spinal muscular atrophy.
Although the development of DNA hydrogels is still in its infancy, McGill researchers have opened up exciting possibilities for biomedical applications of DNA hydrogels by making them a little less rigid—and their uses a little more flexible.